Concept
Our basic concept is to make a kind of nano-swarm robot system called “Bit Clot” that creates scabs to stop bleeding as needed by artificially mimicking aggregation non-linearly according to the amount of signal DNA as a new treatment for hemophilia and VWD (von Willebrand disease).
When bleeding occurs, a signal molecule is released into the blood vessel, then the linker is amplified only when the amount of the signal molecule exceeds a certain value, and finally the aggregation occurs making scab by the linker. It is necessary to cause non-linear responsive aggregation depending on the amounts of linkers.
In other words, we need to design the system that when the amount of signal is below a certain level, the linker does not amplify (it is decomposed even if amplified), and when it is above a certain level, the linker is created, leading actual scab.
DNA robots
To make a system of clotting, We prepared beads, as the body of the aggregation robots, which are attached with DNA strands like the shape of sea urchins. The beads are covered with streptavidin, a kind of protein which causes strong cohesion to biotin, one of the vitamins. We designed two heterogeneous beads, D1 and D2 that are composed of streptavidin beads, unit1 strands and unit2 strands. For beads D1, biotin is jointed to 5’ end of DNA strand (D1 unit1). On the other hand, for D2, biotin is jointed to 3’ end of DNA strand (D2 unit1) and each biotin makes cohesion with streptavidin on the surface of beads. D1 unit2 and D2 unit2 have complementary sequences of each unit1 and linker DNA.
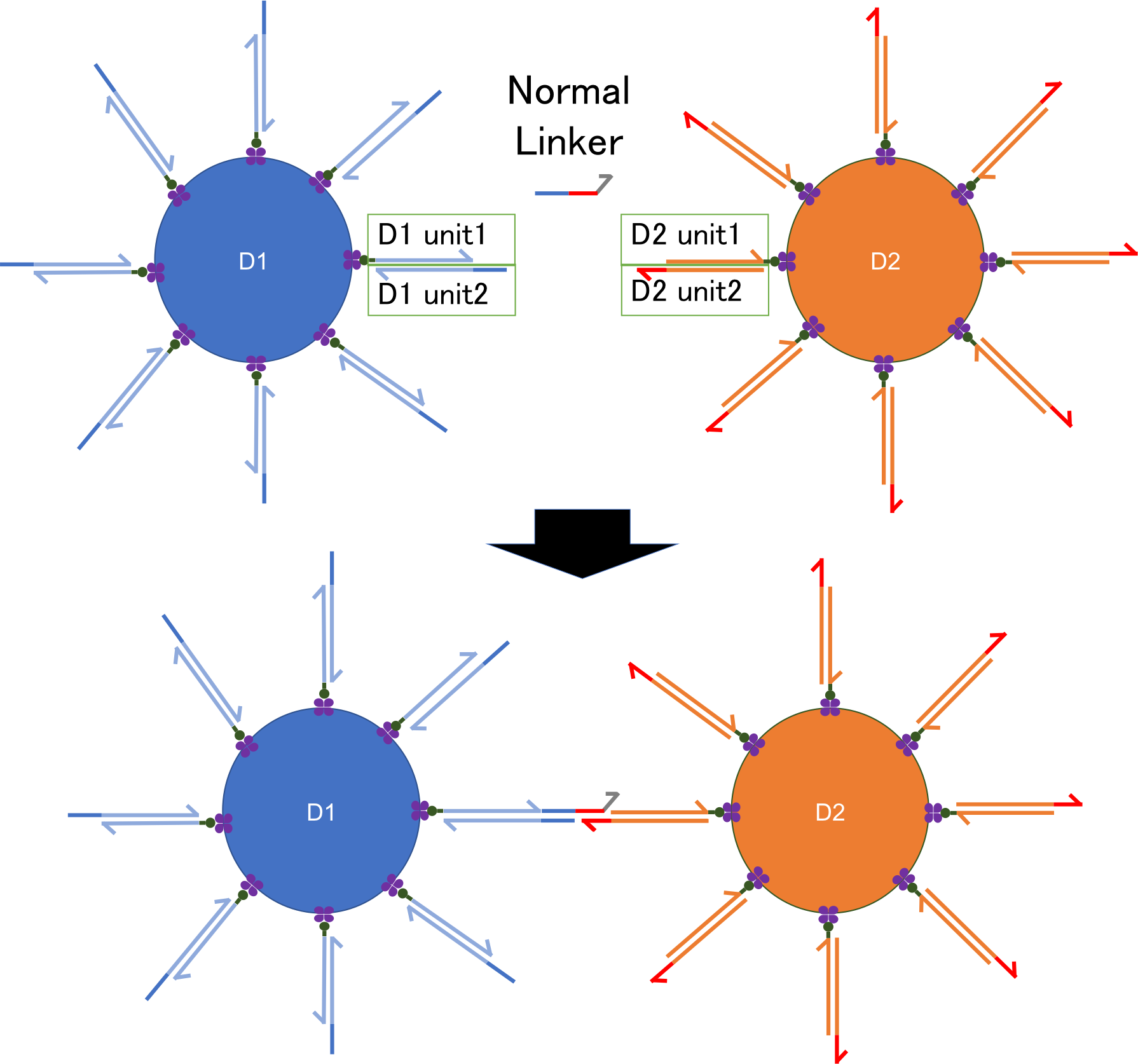
Fig.1 Design of DNA nano-robot
DNA strands of one bead are bound to other DNA strands of another bead by linker DNA strands.
As the directions of DNA strands from beads (5’→3’ or 3’→5’) are opposite to each other, one linker DNA strand can connect D1 strands and D2 strands. Thus, like a bridge connects two lands, linker DNA strands gather the beads making double-stranded DNA where fluorescent substance binds, and observing strength of the fluorescence, which indicates how many beads gather together, we can estimate the amount of linker DNA strands.
PEN DNA toolbox
To implement the two mechanism regarding linker amplification (the amplification of linker by the signal DNA and the decomposition of linker when the signal DNA is under a certain level) into Nanorobot, we utilized PEN DNA toolbox. PEN DNA toolbox is the set of chemical reactions composed of three enzymatic activities; polymerase extension, nickase nicking and exonuclease degradation (Fig.3). In previous reports, PEN DNA toolbox has achieved to show interesting kinetics, such as predator-prey oscillation and bifurcation switch[1][2].
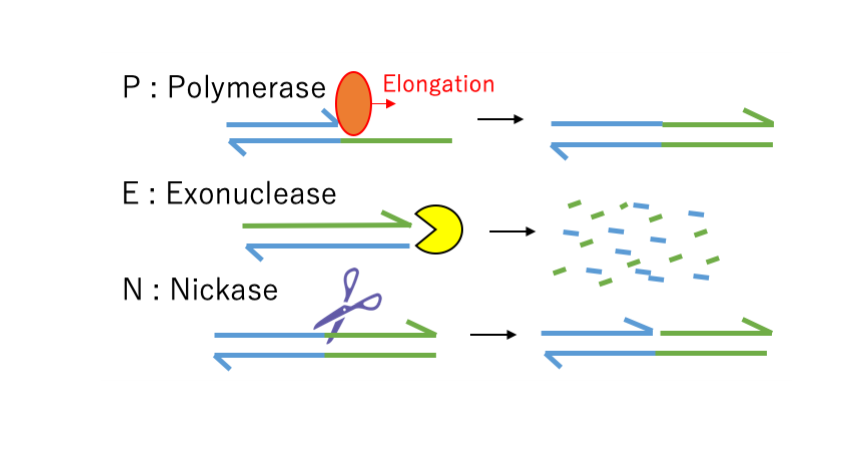
Fig.2 Basic chemical scheme of PEN DNA toolbox
As one of representative module of kinetics, conversion reaction, which is the chemical reaction flow that produces output strands when the input strand presents. To assemble this reaction, we prepare polymerase, nickase, input strand and template which is complementally to input strand and output strand. When the input strand hybridizes the template, input is elongated from 3' and output DNA part is synthesized. After polymerization, nickase recognized specific sequences between the input strand and output strand, after that it cuts and divide into input and output. Finally output strand is separated from template and new output is synthesized one after another.When the input and output are the same sequences, this reaction is called autocatalytic amplification. It highly accelerates the increase of signal because the input amplification in this case is exponential. This autocatalytic amplification greatly helps to detect low concentration molecules.
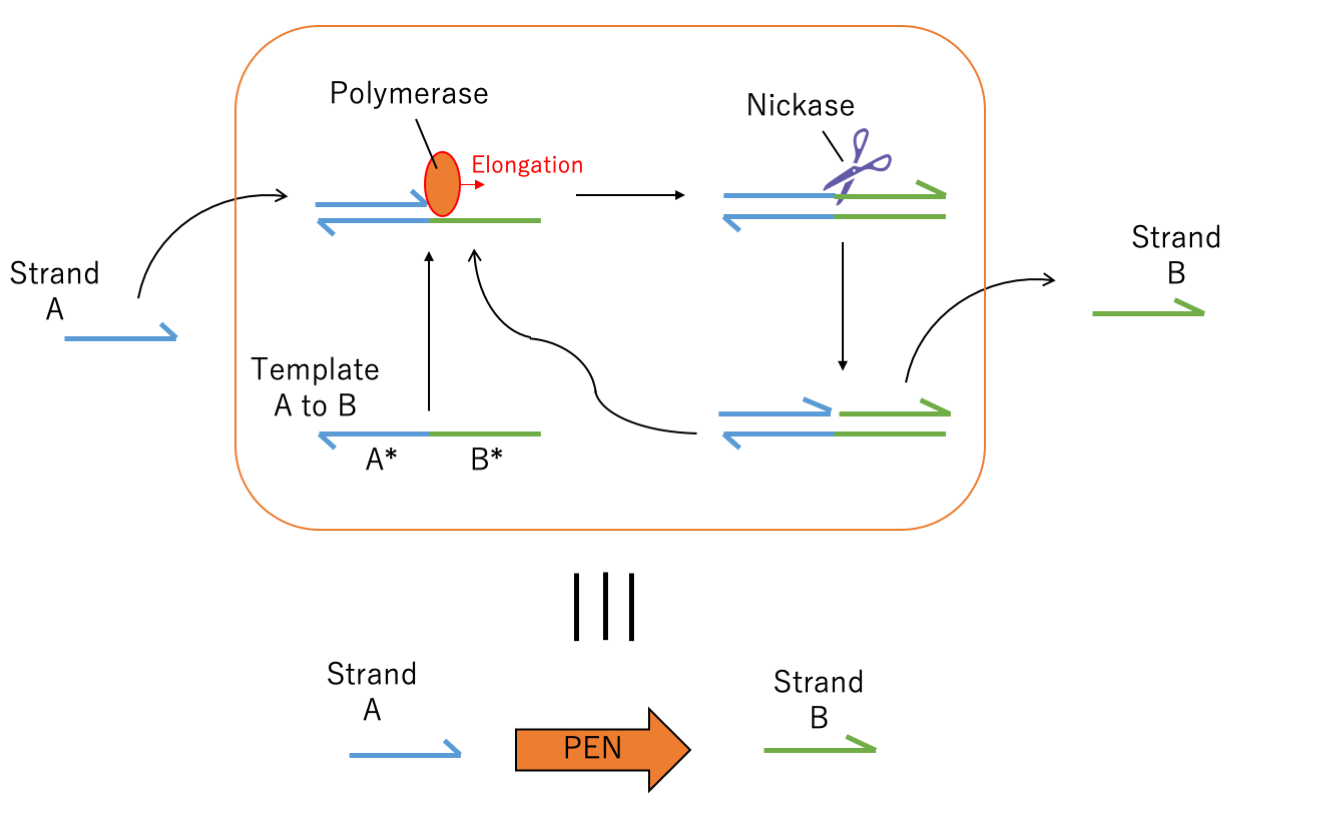
Fig.3 Module of conversion (when A and B are the same sequences, this figure illustrates the module of autocatalytic amplification)
Nano robot system
First, as shown in Fig.4, we designed normal linker. We combined beads D1, D2, strands α, single linker strands, sequences complementary to strands α and linker, and PEN. A complementary strands consists from two kind of strands, that is, from 3’end to 5’ end, complement to α, and then complement to linker.
Thanks to PEN, we can conduct amplification of strands in room temperature and also dissemble the stands. Strand α connect to the complementary strands as primer, and a new strand, whose array is same to that of single linker, is synthesized. Then nickase separates the new array same to α from the strand α, the strand α remains to the complementary strand and the new single linker leaves.
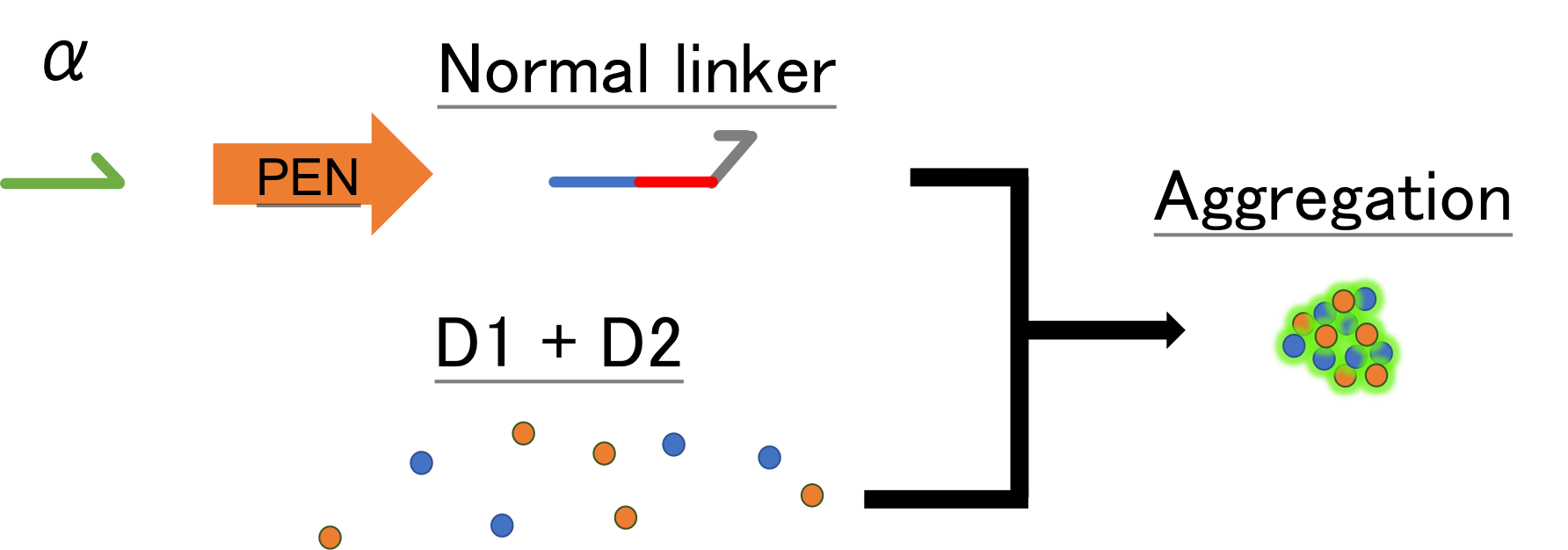
Fig.4 PEN and nano-beads aggregation
Creating threshold by PEN DNA toolbox and drain
we combined two mechanisms, exonuclease and drain (a kind of inhibitor), to degrade the amplified linker DNA and prevent aggregation when the signal is below a certain value,
Exonucleases, which is included in the PEN DNA toolbox, degrade the synthesized linker DNA. The degradation rate increases in proportion to the reciprocal of concentration until all exonucleases bind to the substrate and then becomes constant (c.f. Michaelis-Menten graph). In other words, the graph of the degree of inhibition of exonuclease (y) and the increase in linker (x) is convex when the concentration of α strand is below a certain value (the concentration of α strand at which the synthesized linker binds exonuclease one-on-one), and above the value it stays constant.
On the other hand, the drain inhibits the synthesis of linker from α by competitively binding to α (competitive inhibition). Therefore, the graph of the drain inhibition degree (y) and the concentration of α (x) increases as more α is present until all drains bind to α (the drain concentration is constant in this experiment).
When these two types of inhibitors are combined, the graph of the degree of inhibition (y) with respect to the concentration of α (x) has a greater slope than a straight line until α reaches a certain value (threshold value) C, and when α reaches C, a straight line can be drawn from there.
The value of this switch, C, is adjusted by the two kinds of inhibitors and the concentration of α. For a linear increase of α, in a signal below C, it is adjusted to make the linker increase lower than the inhibition degree (blue part in the image), and in a signal above C (the red part in the image), it is adjusted to make the linker increase higher than the inhibition degree. This makes it possible to realize a system in which linker DNA increases rapidly only when the signal is higher than C.
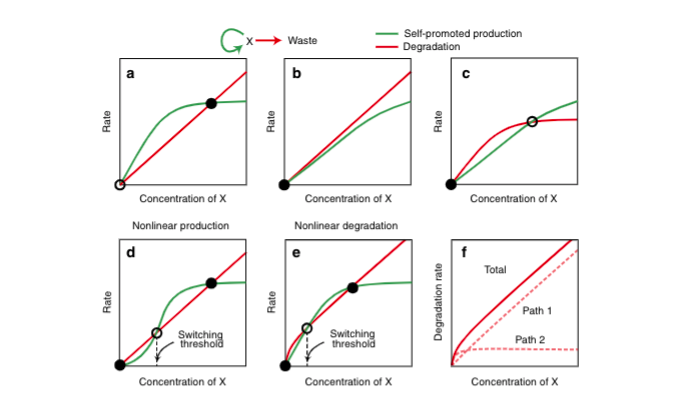
Fig.5 The mechanism of creating threshold (The graph is taken from reference [2])
Drain adjustment
In order to adjust the inhibitor appropriately and clarify the switching at threshold C, it is necessary to adjust the point at which the degree of inhibition for signal α switches from a curve to a straight line in the graph of the degree of inhibition.
By changing part of the linker α sequence, we changed the ease of binding between α and drain, and make it clear for a certain concentration of drain to observe the threshold C for aggregation by α to linker. Specifically, by removing several bases close to the end of the sequence, utilizing the fact that the binding affinity between α and drain decreases as the number of bases deleted increases, we searched for the better linker α sequence that could have a well-defined threshold C at low concentrations. This makes it possible to artificially manipulate the on and off of linker amplification in many environments.
With the combination of the above tools, when the signal DNA that promotes blood clotting is below the threshold, the linker amplified by the action of drain and exonuclease is decomposed and the beads does not aggregate, and only when the signal is above the threshold, the linker DNA is amplified, leads beads aggregation, and makes it possible to make a clot.
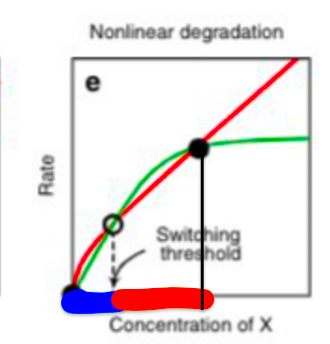
Fig.5 The rough graph of drain adjustment (This graph is taken from reference [2])
Reference
[1] Teruo Fujii and Yannick Rondelez, "Predator–Prey Molecular Ecosystems" ACS nano 2013
[2] Kevin Montagne, Guillaume Gines, Teruo Fujii & Yannick Rondelez "Boosting functionality of synthetic DNA circuits with tailored deactivation" Nature communication, 2016